diff --git a/mkdocs.yml b/mkdocs.yml
index 12ce6b191d4ab858b92b81d5183411a1ffa8cdc9..4b578d616ecfcf8233fab7db82413332cd7e6630 100644
--- a/mkdocs.yml
+++ b/mkdocs.yml
@@ -46,6 +46,7 @@ markdown_extensions:
- admonition
- pymdownx.details
- abbr
+ - footnotes
extra_javascript:
- 'https://cdn.plot.ly/plotly-latest.min.js'
diff --git a/src/1_einstein_model.md b/src/1_einstein_model.md
index 9f2a291f19d71f28f7cd3491d4402a1afa345ca7..945b37eb735e4d9b80b8666eafc532385af146be 100644
--- a/src/1_einstein_model.md
+++ b/src/1_einstein_model.md
@@ -10,9 +10,7 @@ from common import draw_classic_axes, configure_plotting
configure_plotting()
```
-# Lecture 1 – Phonons and specific Heat
-_(based on chapter 2 of the book)_
-Exercises: 2.3, 2.4, 2.5, 2.6, 2.8
+_(based on chapter 2.1 of the book)_
!!! summary "Learning goals"
@@ -22,11 +20,50 @@ Exercises: 2.3, 2.4, 2.5, 2.6, 2.8
- Compute the expected particle number, energy, and heat capacity of a quantum harmonic oscillator (a single boson)
- Write down the total thermal energy of a material
-### Einstein model
-Before solid state physics: heat capacity per atom $C=3k_{\rm B}$ (Dulong-Petit). Each atom is (classical) harmonic oscillator in three directions. Experiments showed that this law breaks down at low temperatures, where $C$ reduces to zero ($C\propto T^3$).
+## Einstein model
+
+### Classical limit
+
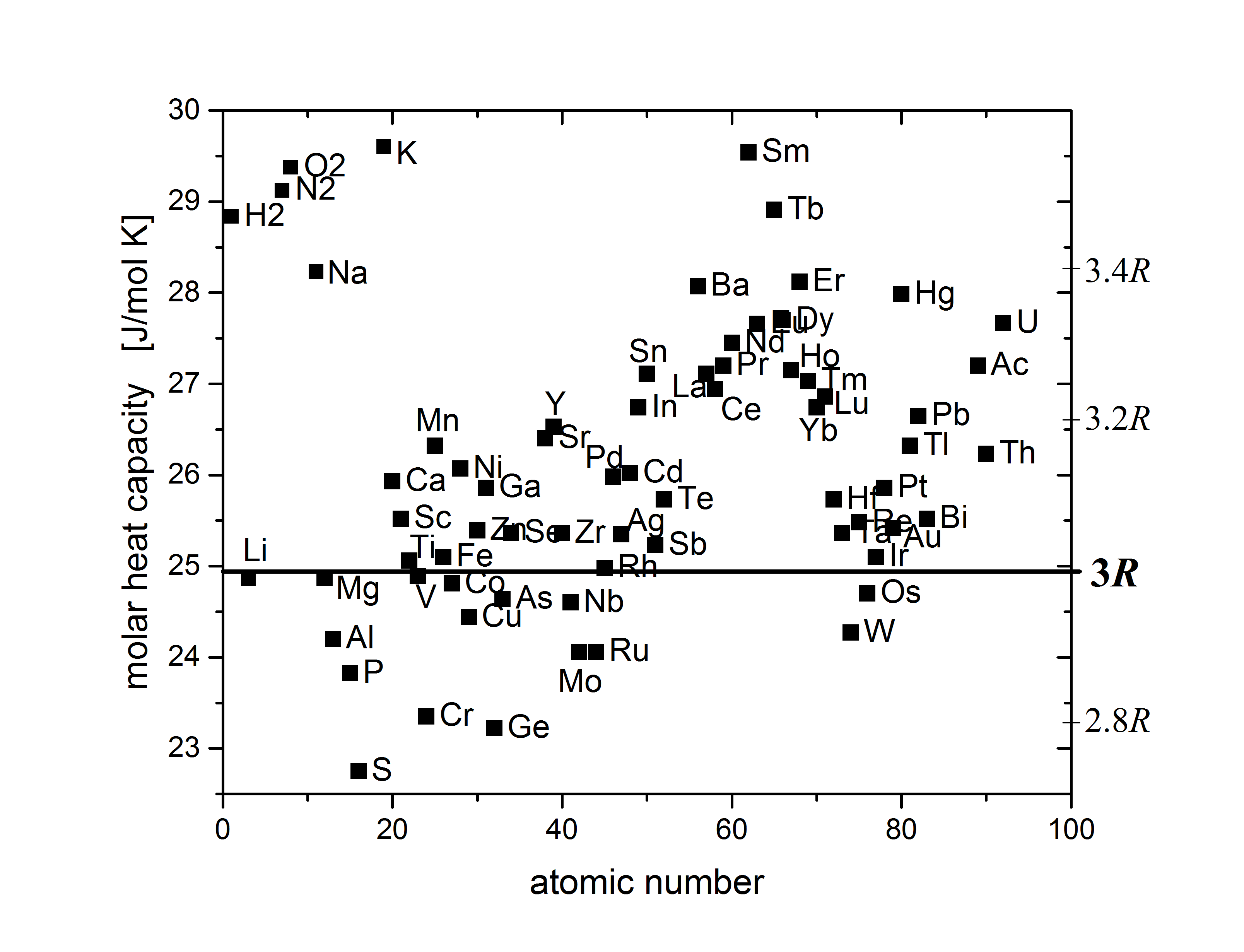
+An empirical observation:
+
+> The law of Dulong-Petit (1819): In most materials heat capacity per atom $C \approx 3k_B$
+
+
+
+??? info "source"
+
+ By Nick B. - Own work, [CC BY-SA 4.0](https://creativecommons.org/licenses/by-sa/4.0 "Creative Commons Attribution-Share Alike 4.0"), [Link](https://commons.wikimedia.org/w/index.php?curid=54031939)
+
+To explain assume that each atom is a classical harmonic oscillator in 3D.
+This gives 6 degrees of freedom, each contributing $k_B/2$ to the heat capacity.
+
+### Problem
+
+Experiments showed that this law breaks down at low temperatures, so Einstein was facing the mystery of this measurement:
+
+```python
+# Data from Einstein's paper
+T = [222.4, 262.4, 283.7, 306.4, 331.3, 358.5, 413.0, 479.2, 520.0, 879.7, 1079.7, 1258.0]
+c = [0.384, 0.578, 0.683, 0.798, 0.928, 1.069, 1.343, 1.656, 1.833, 2.671, 2.720, 2.781]
+
+fig, ax = pyplot.subplots()
+ax.scatter(T, c)
+ax.set_xlabel('$T[K]$')
+ax.set_ylabel('$C/k_B$')
+ax.set_ylim((0, 3));
+```
+
+So there is:
+
+* Strong temperature dependence of $C$
+* At high temperature the law of Dulong–Petit works
+* At low $T$, $C \rightarrow 0$
+
+### Quantum oscillator
This can be explained by considering a _quantum_ harmonic oscillator:
-
+
+
$$\varepsilon_n=\left(n+\frac{1}{2}\right)\hbar\omega$$
@@ -98,7 +135,7 @@ draw_classic_axes(ax)
The dashed line signifies the classical value, $k_{\rm B}$. Shaded area $=\frac{1}{2}\hbar\omega$, the zero point energy that cannot be removed through cooling.
-This is for just one atom. In order to obtain the heat capacity of a full material, we would have to multiply $C$ (or $\bar{\varepsilon}$) by $3N$, _i.e._ the number of harmonic oscillators according to Einstein model.
+This is for just one atom. In order to obtain the heat capacity of a full material, we would have to multiply $C$ (or $\bar{\varepsilon}$) by $3N$, _i.e._ the number of harmonic oscillators according to Einstein model.[^1]
```python
# Data from Einstein's paper
@@ -136,7 +173,7 @@ $$
Z = \int_{-\infty}^{\infty}dp \int_{-\infty}^{\infty} dx e^{-\beta H(p,x)}.
$$
2. Using the solution of 1., compute the expectation value of the energy, and the expectation value of .
-3. Compute the heat capacity. Check that you get the law of Dulong-Petit but with a different prefactor.
+3. Compute the heat capacity. Check that you get the law of Dulong-Petit but with a different prefactor.
4. Explain the difference in the prefactor by considering the number of degrees of freedom.
@@ -165,4 +202,6 @@ Naturally occurring lithium has [two stable isotopes](https://en.wikipedia.org/w
1. Assume that the strength of the returning force $k$ experienced by each atom is the same. What is the difference in the oscillation frequencies of different isotopes of lithium in the lithium crystal?
2. Write down the total energy of lithium assuming that all $^6$Li atoms are in $n=2$ vibrational state, and all $^7$Li atoms are in $n=4$ vibrational state.
3. Write down the total energy of lithium at a temperature $T$ by modifying the Einstein model.
-4. Compute the heat capacity of lithium as a function of $T$.
\ No newline at end of file
+4. Compute the heat capacity of lithium as a function of $T$.
+
+[^1]: The data in this plot is the same as what Einstein used, but the curve in this plot is improved compared to what Einstein did, see [this blog post](https://quantumtinkerer.tudelft.nl/blog/footsteps-of-einstein/) for the backstory.
diff --git a/src/2_debye_model.md b/src/2_debye_model.md
index b0157ac3405104ef27c45d9c5b3344490bc50771..f97c2883b8619f9e5db41cc1492c6918cef59950 100644
--- a/src/2_debye_model.md
+++ b/src/2_debye_model.md
@@ -10,6 +10,8 @@ from common import draw_classic_axes, configure_plotting
configure_plotting()
```
+_(based on chapter 2.2 of the book)_
+
!!! summary "Learning goals"
After this lecture you will be able to:
@@ -170,7 +172,7 @@ Explain your answer.
4. At low $T$, show that $C_V=KT^{n}$. Find $n$. Express $K$ as a definite integral.
### Exercise 3: Different phonon modes
-During the lecture we derived the low-temperature heat capacity assuming that all the phonons have the same sound velocity $v$.
+During the lecture we derived the low-temperature heat capacity assuming that all the phonons have the same sound velocity $v$.
In reality the longitudinal and transverse modes have different sound velocities (see [Wikipedia](https://en.wikipedia.org/wiki/Sound#Longitudinal_and_transverse_waves) for an illustration of different sound wave types).
Assume that there are two types of excitations:
@@ -188,4 +190,4 @@ How does this change the Debye result for the heat capacity?
??? hint
- Write down the total energy as an integral over $k$, then change the integration variables so that the spherical symmetry of the integrand is restored.
\ No newline at end of file
+ Write down the total energy as an integral over $k$, then change the integration variables so that the spherical symmetry of the integrand is restored.